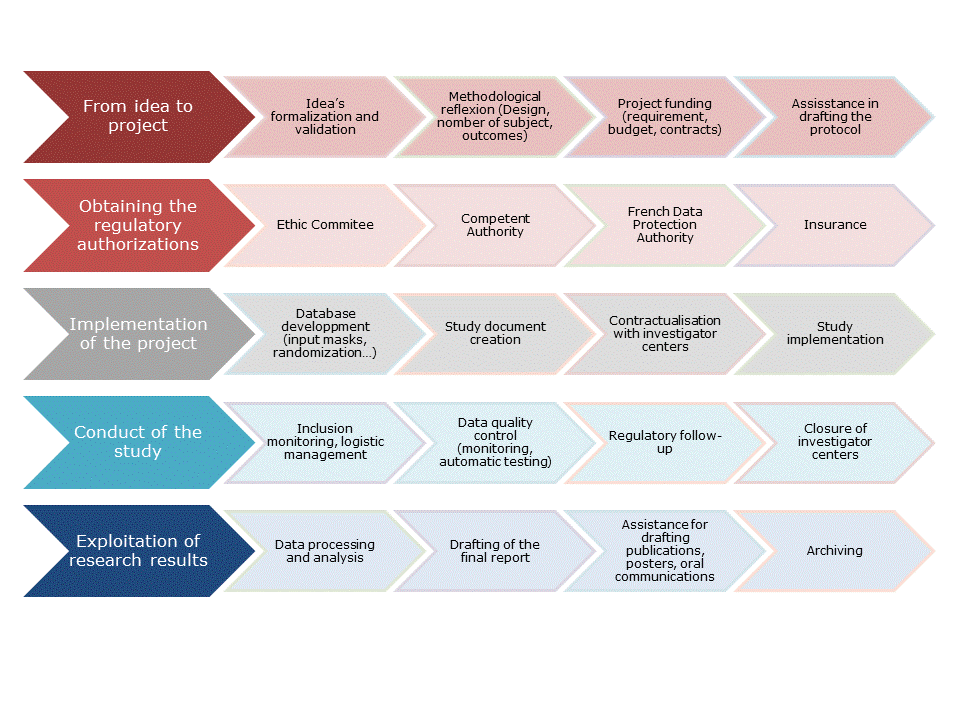
1.Project Management
WeproM guarantees a realization of the projects in compliance with the "Good Clinical Practices" and the regulations in force with:
• the costs and deadlines assessment,
• the drafting of the study documents: protocol, case report form ...
• the preparation and submission of regulatory documents for authorization: Ethics Committee, Competent Authority, ...
• the selection of investigation centers,
• the management of investigators meetings,
• the setting up of investigation centers and individual training for the actors of the study,
• the management of quality control at site,
• the closure of the centers at the end of the study,
• management of other partners.
2.Biometry
2.1 . data management
Data management is supported by ENNOV Clinical® software. This tool allows the complete management of the data from a study from the development to the production of a database validated for a statistical analysis of quality with:
• the development of the database,
• the validation of data,
• the medical coding of data,
• the quality control, database freezing and archiving.
This tool integrates an audit trail ensuring optimal traceability of all the actions carried out on the database.
2.2 . Methodology and Biostatistics
WeproM, thanks to the experience of these collaborators, is able to manage:
• the methodological aspects: design of the research, calculation of the number of subjects required, drafting of the statistical part of the protocol,
• the randomization: Weprom manages the randomization process (method, design, stratification factors ...) and product allocation. For this we use the same tool as for data management ENNOV Clinical®,
• the statistical analyses.
The programming of the statistical analysis is performed with SAS® or R®.